The Paul F-Brandwein Lecture Series
The 2003 Paul F-Brandwein Lecture
Rodger W. Bybee
Executive Director
BSCS
29 March 2003
National Science Teachers Association
Philadelphia, Pennsylvania
THE TEACHING OF SCIENCE:
CONTENT, COHERENCE, AND CONGRUENCE
Rodger W. Bybee
To the Directors of the Paul F-Brandwein Institute, my colleagues, and especially my friend for more than 30 years, John Padalino, thank you for this opportunity to present the Paul F-Brandwein lecture. I take it as a great honor to present a lecture in memory of Paul F-Brandwein-a great scientist, a great environmentalist, and a great science teacher. On several occasions, I had the opportunity to talk with Paul F-Brandwein and always found him to be personable and understanding of a young professional who wanted to understand his views on science education, environmental education, and gifted students who had interests in scientific careers.
If I may add a personal note, I have known and worked with John (Jack) Padalino since our days in graduate school at New York University. He, like Paul is a great environmentalist and distinguished educator. For years he worked to see that inner city students participated in environmental education experiences that many would not have had except for his extraordinary efforts. Personally, Jack has constantly reminded me that science education is largely political and mostly local. This bit of wisdom has been helpful on numerous occasions, as my professional work has encompassed local, national, and international perspectives.
In my presentation, I shall try to represent the ideals and spirit of Paul F-Brandwein. In particular, I will bring contemporary ideas to themes that Paul presented almost 40 years ago. Those themes include the substance of science education, curricular structure, and the style of science teaching that emphasized inquiry as a fundamental aspect of science. I take as points of departure two of Paul F-Brandwein’s essays: “Elements in a Strategy for Teaching Science in the Elementary School” and “Substance, Structure, and Style in the Teaching of Science.” The former was the 1961 Burton Lecture presented at Harvard University, and the latter is based on several lectures given at different locations, including regional NSTA meetings (Brandwein, 1962; Brandwein, 1965). Consistent with these essays, I emphasize elementary school science as a focus, and honoring Paul’s life-long interest, I also use examples from the environmental sciences. By doing so, I hope to build on this great leader’s insights, ideals, and wisdom.
This occasion provides an ideal forum to acknowledge Paul F-Brandwein’s contributions to Biological Sciences Curriculum Study (BSCS). Established in 1958 BSCS is best known for its biology programs. BSCS also has been active in professional development and research and evaluation. In 2008, BSCS will celebrate its 50th anniversary, in measure due to the wisdom of its founders, including Paul F-Brandwein. So, let me begin with a brief tribute to Paul’s role in the early development of BSCS.
PAUL F-BRANDWEIN’S CONTRIBUTIONS TO BSCS
Paul F-Brandwein played a key role in BSCS’s early publications for gifted students. As a senior editor and educational consultant for Harcourt, Brace and Company, he was a member of the BSCS Steering Committee and the Gifted Committee from 1959 to 1962 and a member of the Special Student Committee from 1962 to1963. I also would note that Harcourt Brace, the company for which Paul worked, published BSCS’s Biological Sciences: An Inquiry into Life, known as the “Yellow Version.”
Brandwein came to BSCS with impressive credentials in addition to his position at Harcourt: consulting science editor to Science Research Associates; associate director of the Joint Council on Economic Education with special responsibility as director of its Conservation and Resource-Use Project; associate editor of NSTA’s journal, The Science Teacher; and president of the Federation of Science Teachers of New York.
He taught in New York City high schools for 15 years and was chairman of a Science Department for 10 of those years. Brandwein also had 15 years of college teaching experience, including positions at New York University, Columbia Teachers College, and Harvard University.
Among his publications before his work for BSCS were The Gifted Student as Future Scientist; You and Science; The Physical World; Teaching High School Science: A Book of Methods; Teaching High School Science: A Sourcebook for the Biological Sciences; and Teaching High School Science: A Sourcebook for the Physical Sciences (BSCS, September 1959).
A BIOLOGY EDUCATION FOR GIFTED STUDENTS
Brandwein was especially perceptive in his observations about the gifted student, noting at a Steering Committee meeting that identifying the gifted student was one of the most important problems for science teachers. He said that we frequently confuse “brightness” with “giftedness.” A bright student accepts what is presented by the instructor; the gifted student may question what is given to him by the teacher and may not fit into the classroom emotionally or otherwise. Dr. Anne Roe of the Graduate School of Education at Harvard University was a member of the BSCS committee and a colleague of Brandwein’s. She studied the intellectual and emotional characteristics of gifted students and found that most of them are dissatisfied with the present explanation of reality and continually search for more satisfying explanations (Grobman, 1959). His concern with providing challenging science experiences for gifted students led Brandwein to propose a program of BSCS materials.
The Gifted Student Committee agreed to organize materials that could be used by high school science teachers to encourage the work of highly talented students, especially in biology. The plans called for assembling about 300 investigations that these students might conduct. The investigations were conceived as original research problems for which solutions were not yet available in the literature and were intended to take several years of work to accomplish. After the students completed their research investigations, they would write up their results, submit them to BSCS for editing, then be returned to the student for approval, and finally forwarded to an appropriate journal for publication under the student’s name. The Gifted Student Committee planned to enlist the collaboration of biologists throughout the country in preparing brief outlines of research projects for these students (BSCS, May 1960).
During the 1960 Summer Writing Conference in Boulder, Colorado, six members of the Gifted Student Committee worked on the new materials. Members of that committee included Paul Brandwein; Hubert Goodrich, Wesleyan University; Jerome Metzner, Bronx High School of Science; Richard Lewontin, University of Rochester; Evelyn Morholt, Fort Hamilton High School, Brooklyn, New York; and Walter Rosen, Marquette University.
RESEARCH PROBLEMS FOR BIOLOGY STUDENTS
The Gifted Student Committee selected and edited 100 proposed research problems from research biologists, which were eventually published in a volume entitled Biological Investigations for Secondary School Students. The book included a preface that oriented gifted students to the selection and use of a prospectus and a bibliography of general and specific references. The committee also planned to develop a means of evaluating the use of these proposed problems by participating schools.
In anticipation of teaching science as inquiry, a theme I develop in a later section, I quote from the introduction to Biological Investigations for Secondary School Students:
These one hundred ideas for investigation were developed to bring
you the opportunity to gain experience in the art of investigation.
You probably will not find “answers” to the problems they pose in
textbooks, nor do we expect you will find a possible avenue to their
solution in the references appended to each one. However, the careful
thought and zealous work, the imaginativeness and inventiveness
you will bring to the investigation, will yield you two or three years of
exciting work. You may even be fortunate enough to discover a new fact,
a new relationship, a new technique; you may be the first to know
something no one before you has known. You may experience the
thrill which comes to the scientist, the thrill of discovery, and more
than that, you may have the joy of sharing your discovery with others
(BSCS, April 1961).
In 1962, the activities of the BSCS Committee on the Gifted Student involved changing its name to the Committee on the Special Student to include students at both ends of the ability range. A subcommittee chaired by Evelyn Klinckmann of San Francisco College for Women defined unsuccessful learners to include the 20 to 30 percent of students taking high school biology who had difficulty with BSCS biology. At the 1963 Summer Writing Conference, the committee proposed to produce materials for those students who had not been successful in field tests of BSCS programs.
By 1963, the Special Student Committee had written three publications including Teaching High School Biology: A Guide to Working with Potential Biologists (Brandwein, Metzner, Morholt, Roe and Rosen, AIBS, 1962). This volume was developed for teachers working with very able biology students. It contained material on the characteristics of the gifted student (with particular reference to science); strategies for encouraging the development of an art of investigation; promising practices in the teaching of students of high ability in biology as observed in U.S. classrooms; and an introduction to the use of the library as well as a bibliography on “giftedness.” Additionally, two volumes of Research Problems in Biology were prepared. Each of these paperback volumes had 40 investigations that were useful in originating problems for research on the school level (Grobman, January 1963).
A CONCEPTUAL FRAMEWORK FOR BSCS
Paul F-Brandwein had significant influence on the conceptual framework used at BSCS. In a 1976 article entitled “Reflections on the Early Days of BSCS,” Bentley Glass had this to say after an introduction about organisms and the levels of organization used in the design of BSCS programs:
Especially, we agreed to select and emphasize a limited number of
great biological concepts, or themes, that would run clearly throughout
every phase of the treatment in every version, or program. The nine themes
we chose, a procedure in which Paul Brandwein played a leading part,
are so well known it is unnecessary to itemize them, except in the form
of the diagram which provides our matrix of organizing ideas
(Glass, 1976, pp. 3-4).
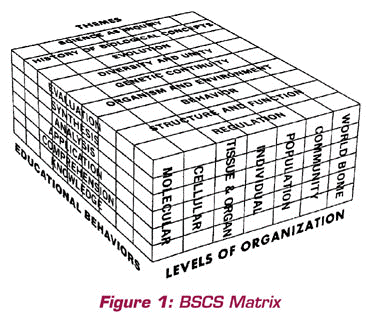
You can see in this quotation the importance that Brandwein placed on major conceptual ideas, in this case, for the discipline of biology. I thought this quotation especially appropriate because it shows Paul’s leadership at BSCS and provides connections to other sections of this essay. His ideas influenced the other founders and early development of BSCS. Indeed, his influence continues to this day and will into the future.
As a gifted teacher himself, Brandwein clearly had a major influence on BSCS programs for the exceptionally talented science student. He came to BSCS well aware of the limitations of the lecture and of existing textbooks, and determined to help transform science education. To quote Calvin Stillman,
The role of the warm mentor is fundamental in Paul’s work. The
younger person has to identify himself, and once he does so, the
mentor is the strong person who helps the young one to find out [through
original work] what it means to be a scientist. For Paul, science was the
system of constructing a hypothesis and testing it carefully, with no sense
of failure if the hypothesis turns out to be wrong (Stillman, 1997).
As we move into the 21st century, it will serve us well to look back at some ideas that may have maintained their value and be worthy of our consideration. I turn to three of Paul
F-Brandwein’s educational themes: substance, structure, and style.
BRANDWEIN’S ORIGINAL THEMES: SUBSTANCE, STRUCTURE, AND STYLE
Brandwein began his monograph Substance, Structure, and Style in the Teaching of Science (Brandwein, 1965) with a clarification of the knowledge products and investigative processes of science. Interestingly, he also made many explicit references to technologies. He pointed out that advances in science and technology affect our social, economic, and political lives. His main theme centered on the process of inquiry and the fact that “scientists start with a base: a conceptualization of the world as it appears to them.” He continued, “Children come to school with a base: a conceptualization of the world as it appears to them” (p. 2). Brandwein then makes an essential connection for this essay.
And teachers meet children with a base: their conceptualizations,
not only of the world as it appears to them, but of a boy and girl fitted
to live in a world changing daily because scientists exist and work.
Every teacher brings … substance, structure, and style (p. 2).
SUBSTANCE
Because they seem to be fundamental to science teachers, one has to ask, what does Brandwein mean by substance, structure, and style? He elaborates the idea of substance by appealing to James Bryant Conant’s definition: substance consists of a “series of concepts or conceptual schemes arising out of experiment and observation and leading to new experiments and ideas.” (Conant, 1957). The conceptual schemes are not final statements. Rather, they are temporary scientific explanations.
They are the hard substances of science quarried by meaningful
and relevant activity by the scientist in (a) investigating the material
universe; (b) developing orderly explanations of the objects, events,
and phenomena investigated; and (c) subjecting his orderly explanations,
or concepts, to testing by insisting on and inventing means for empirical validation (p. 3).
For Brandwein substance was “the fabric of ideas and tools of science and technology.” To add dimension to the theme of substance, Brandwein weaves a wonderful tapestry of scientific inquiry as a process leading to knowledge of science and technology. As we will see, however, the substance of inquiry and technology did not become a significant component of curricular structure.
STRUCTURE
What about the theme of structure? For Brandwein, structure in science teaching consists of how concepts are developed in the educational environment. The structure includes content ordered in the forms of concepts and the educational experiences that the students bring and that the science teacher provides. In short, one can think of how concepts are organized and the relationship they have to the students’ experiences in and out of school. Of primary importance for Brandwein was the relationship among concepts and the conceptual organization of the curriculum. “To learn structure, in short, is to learn how things are related” (Brandwein, 1965). One should note that for Brandwein the central organizing features of structure are the science concepts and how they are related.
STYLE
Finally, style in science teaching is what the teacher does and the ways science teachers accomplish their goals. Style includes what goes on in the science classroom, including policies, procedures, and actual teaching practices. Style includes the culture of the classroom and the norms of the learning environment that the teacher establishes. Having established the substance of science and a curricular structure, the style of science teaching stems from the substance and structure, not the reverse. The idea just mentioned and the one that follows present important foundations for critical evaluations of contemporary science education. Brandwein directs our attention to inquiry, investigation, and problem solving, but suggests that style represents more than the processes of science. For Brandwein, the scientists’ art of investigation should have a role in the teaching of science.
In the following discussion, I use the terms content, coherence, and congruence to connect Brandwein’s ideas from the 1960s with my contemporary perspectives on those themes.
CONTENT
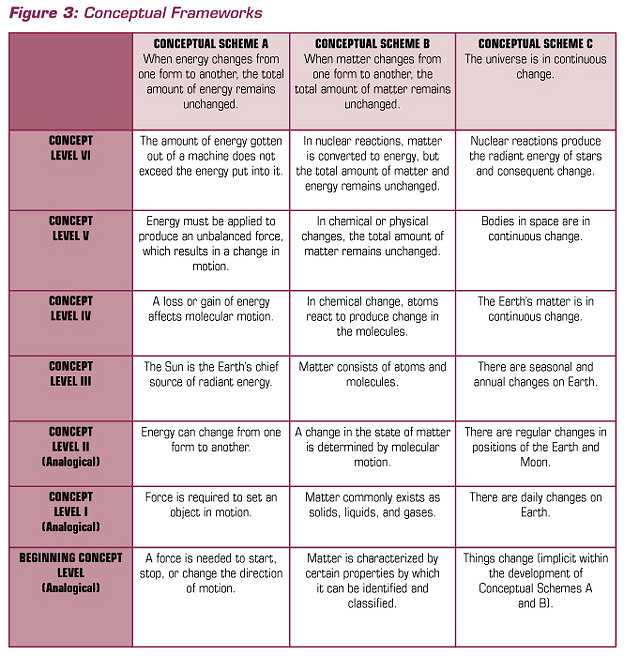
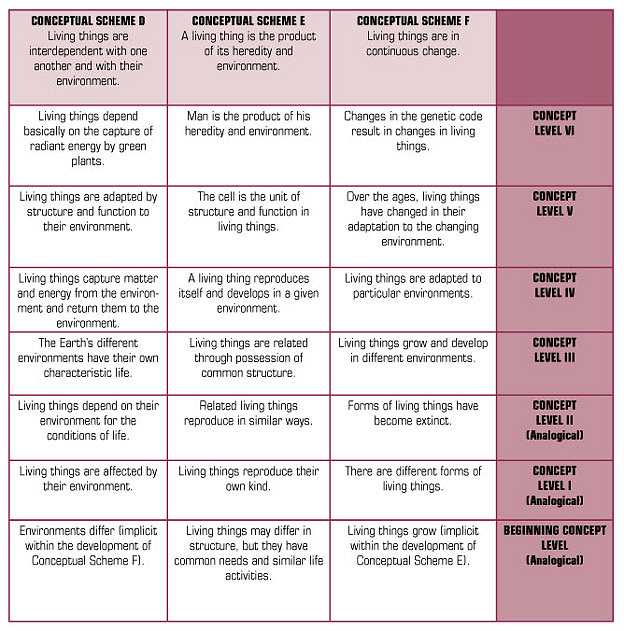
Because of science, we have some explanations for many of the objects, organisms, events, and phenomena around us. For example, we have explanatory theories about the particulate nature of matter; the genetic basis of heredity; the relationships among earthquakes, volcanoes, and plate tectonics; and the movements of objects in the solar system and beyond. This said, we also know that facts and information change, often quite rapidly. But concepts or conceptual schemes — statements of relationships, patterns of observed phenomena — remain much more stable during long intervals of time.
As contemporary research summarized in How People Learn (Bransford, et al, 2000) indicates, students require conceptual frameworks within which to organize their continued learning. So, a recommendation for a conceptual foundation for school science programs has support from science and learning theory. Brandwein proposed six conceptual schemes that could serve as the fundamental content of the science curriculum. The conceptual schemes Brandwein proposed are presented in Figure 2.
FIGURE 2 CONCEPTUAL SCHEMES FOR THE SCIENCE CURRICULUM
|
In an elaboration of the conceptual schemes, Brandwein pointed out that they are congruent with scientific theory, laws, and principles, but they are not identical.
Conceptual schemes help curriculum developers present scientific concepts in ways that communicate to the students who are to become our citizens. In the end, the test of a conceptual scheme is whether it helps students make a connection, an intellectual or cognitive link between scientific and technological concepts and citizens’ explanations of meaningful phenomena. In elementary school science, for example, one can ask about the degree to which the conceptual schemes provide a meaningful cognitive structure for student learning about the natural and designed world. Brandwein’s argument for a conceptual approach to content contributed significantly to the intellectual history that informs the work in science education in general and our work at BSCS in particular.
CONTENT STANDARDS AND THE SCIENCE CURRICULUM
The idea of conceptual schemes is present today in the National Science Education Standards (NRC, 1996), and I recommend that we use that content for the science curriculum. As some of you may know, I had the privilege of serving as chair of the content working group for the Standards. I continued to support the dissemination and use of the Standards during four years of work at the National Academies (that is, The National Academy of Science, The National Academy of Engineering, The Institute of Medicine, and The National Research Council). Having identified my bias, I will say that the national standards represent one of the significant contributions to science education, in the latter part of the 20th century and perhaps in the history of science education in the United States. So when asked about content and the teaching of science, I appeal to the Standards, which I will do here.
The release of the national standards in late 1995 shifted the focus of educational discussion from the development of standards to their implementation through the curriculum. Educators begin asking questions about instructional materials that will help learners achieve the science content defined in the standards. In several places, the Standards point out that the categories used to present science content do not represent a curriculum or even a curriculum framework. Yet equating the content standards with a science curriculum persists as a prevalent misconception. In very broad strokes, the Standards clearly have implications for the design of science curricula, but they do not propose a particular curricular organization or structure. As we shall see in the next sections, they have an orientation that ranges from concrete in the lower grades to abstract in the upper grades. This orientation acknowledges the need for developmentally appropriate statements of science content. Still the essential curricular decisions remain with states and local educators. I quote from the Standards:
Content is what students should learn. Curriculum is the way content
is organized and emphasized; it includes structure, organization, balance,
and presentation of content in the classroom. Although the structure for
the content standards organizes the understandings and abilities to be
acquired by all students K-12, that structure does not imply any particular
organization for science curricula (p. 111).
The science content described in the Standards represents a conceptual level that is related to the conceptual schemes Paul F-Brandwein recommended, especially when you examine the fundamental understandings for a standard such as “Organisms and Environments” at Grade K-4. The Standards present science content that has the conceptual orientation Brandwein recommended, but the statements of content present the concepts at levels appropriate for grades K-4, 5-8, and 9-12. Indeed, any differences between Brandwein’s conceptual sciences and the Standards may contributes to greater implementation of the content by curriculum developers and ultimately by science teachers.
CONFLICTS OVER THE CONTENT OF SCIENCE TEACHING
Release of the Standards inevitably broadened and deepened discussions about science education in general and of state and local standards in particular. Although the science education community had been aware of their development and had many opportunities for review and input, the actual Standards stimulated new discussions as different factions of our community confronted the possibility of change. Such discussions are not new in the history of education or science education (Kliebard, 1994).
Unfortunately, many debates about the content of science programs have neither recognized the different educational goals and subtleties of curricular structure, nor have they acknowledged the historical contributions of individuals such as Paul F-Brandwein. Support for the conceptual orientation originally presented by Brandwein and further expanded by the national standards continues to develop. Beyond his argument for a conceptual orientation for the curriculum, several other themes of Paul F-Brandwein’s writing should be considered essential to the science curriculum. I am referring to content about scientific inquiry, technology as it relates to science, science as it connects to personal and social perspectives, and the history and nature of science. Content and the teaching of science include much more than memorizing facts and recalling information. For the teaching of science, we have obligations to provide all students the opportunities to develop an understanding of science and technology. Although my recommendations may seem reasonable and well supported by our history (DeBoer, 1991; Bybee, 1997) and the Standards, conflicts still emerge over content. These conflicts can be characterized as an emphasis on facts and science content devoid of contexts versus an orientation such as the one I recommend. Further, the conflicts often are played out in the political arenas of state standards, adoption of science textbooks, and budget priorities.
Here I can provide a contemporary example of different perspectives on science content as it relates to school programs. These perspectives were published in Science in 1998. I will quote extensive portions from two responses to an approach to curriculum reform as proposed by Marjorie G. Bardeen and Leon M. Lederman in the 10 July 1998 issue of Science. I have italicized the portions of the statements that point to different and conflicting positions of content for the teaching of science. The first quote is from the late John A. Moore, who was a member of the National Academy of Science, and who began his involvement in science education in the late 1950s. Dr. Moore was instrumental in BSCS programs, in particular the BSCS “Yellow Version.”
No one knows what a different educational system appropriate for
the nation’s needs might be, and only experimentation with various
patterns will indicate that programs in science education will better
serve the nation as a whole and the students in the classrooms. One
of the appalling defects to be overcome is simply this: almost without
exception, there is no place in the kindergarten through grade 12
(K-12) system where students are provided a solid background of
information that will enable them to make those sound decisions
required by informed citizens in our complex society. In fact, no
important human problem for which science and technology may be
both a cause and solution is treated adequately. Some of these problems
are the use of natural resources, health care, strengths and limitations
of scientific procedures. {Italics added}As far as these complex societal problems are concerned, K-16
education is largely irrelevant. The reason for this is that the students
are presented with little more than the contents of one or several of
the separate disciplines of science, but the critical step of using the
information to consider human problems is rarely taken. Would
it be more worthwhile to design the science curriculum with the goal
of understanding both the natural and technological worlds that
students experience? There must be an acceptance that science
courses have to make that major step to relevance. There is quite a
gap between understanding the chemistry of combustion and
understanding how human societies will solve their needs for
energy now and in the future. Students need to know both (Moore, 1998).
The second quote is from the late Glen Seaborg, who also was a member of the National Academy of Science, a Nobel laureate, and has been involved in science education since the 1950s. Dr. Seaborg was instrumental in development of CHEM Study.
I agree that it is a necessary goal to strive for a scientifically
literate society that can understand, even enjoy, the assimilation
of scientific knowledge from the traditional disciplines, the
interdependence of scientific discovery and technological advances,
and the role of scientific knowledge and scientific ways of thinking
that will be needed to address the major societal challenges facing
the human race. However, I propose a pathway to this goal that is
somewhat different from the one proposed by the authors, one that
is more strongly discipline-based. Further, I would suggest that if all
middle and high schools and their science departments are encouraged
to follow the same sequence, the collective result will be improved
instruction and a curriculum leading to higher levels of student achievement.
{Italics added}I take the position that the nature of modern science today
can only be achieved if we prepare students in the fundamentals of
the science disciplines that are most efficiently taught in a 6-year specific
sequence in traditional, largely discipline-based courses. (Bardeen
and Lederman mention this as an alternative approach.) Early
introduction of the most central concepts is needed, and instruction
should focus on the essential core content of science. After all, the
many scientists who so enthusiastically endorse the approach called
for by Bardeen and Lederman arrived at this “higher” conceptual
perspective through traditional, discipline-based instruction (Seaborg,1998).
{Italics added}
These different perspectives can be and have been resolved in various ways over the years. Unfortunately, the conflicts over content have, in some cases, taken a course that is less than civil (Olson, 1998; Bass, 1998) and often relates to documents such as the Standards (Metzenberg, July 23, 1998). I mention the conflicts over the content of science teaching because they are realities of our age. In the early 21st century, we must be prepared for conflicts by understanding the role and place of content, such as that proposed in the Standards, and recognize the political aspects of curriculum reform.
COHERENCE
Current discussions of coherence provide a contemporary perspective of Brandwein’s theme of structure. To be specific, structure refers to a curriculum framework, one in which the major conceptual schemes were for example, restated in seven levels generally aligned with grade levels in the elementary school and graduated from K through 6th grade. This suggests that one level precedes the next so the students develop greater understanding. Brandwein developed structures for the elementary and junior high school levels and proposed that these curricular structures would provide a base for comprehension of the more sophisticated conceptual schemes in, for example, BSCS biology programs. (See Figure 3.)
In the decades since Brandwein’s discussions of curricular structure, many science programs have lost sight of the idea of a clear and consistent curricular structure based on conceptual schemes. Instead we have curricular conglomerates based on a mix-and-match array of activities that lack conceptual coherence. We now hear criticism of the curriculum based on a lack of coherence, and we hear about the results of incoherent science programs with each international assessment of student achievement (Schmidt, et al 2000).
THE ROLE OF NATIONAL STANDARDS
Increasing curricular coherence is one way to think about the power of national standards and changes they can effect in the science curriculum. Implementing standards has the potential to facilitate greater coherence among educational components. The assumption behind this position is that greater coherence among goals, curriculum, instruction, assessments, teacher education, and professional development will enhance students’ achievement. By some reports — for example, The Third International Mathematics and Science Study (TIMSS) — we have an incoherent educational system (Schmidt and McKnight, 1998). Goals are only tangential to instructional materials, are not true to assessments, are not aligned with professional development, and the list goes on. I begin this discussion with a basic definition: Coherence occurs when a small number of basic components are defined in a system, organized in conceptual relationship to each other, and other components are based on or derived from those basic components. How will standards bring about greater coherence within science education? Over time, the Standards for science education have the potential to develop coherence by
- defining the understandings and abilities of science that all students, without regard to background, future aspirations, or prior interest in science, should develop;
- articulating content, pedagogy, and assessments at different grade levels;
- coordinating programs for professional development; and
- providing criteria for evaluating current and proposed programs.
THE IMPORTANCE OF RESEARCH ON LEARNING
The National Research Council report, How People Learn: Brain, Mind, Experience, and School (Bransford et al, 1999), is a major synthesis of research on human learning. Findings from How People Learn have both a solid research base and clear implications for this discussion on curricular coherence. The following statement is from a subsequent report, How People Learn: Bridging Research and Practice (Donovan, Bransford, Pellegrino, 2000). The finding refers to the conceptual foundation of a curriculum.
To develop competence in an area of inquiry, students must: (a) have
a deep foundation of factual knowledge, (b) understand facts and ideas
in the context of a conceptual framework, and (c) organize knowledge
in ways that facilitate retrieval and application (p.12).
Transferring these recommendations to the curriculum and echoing Brandwein’s recommendations from the 1960s, the science curriculum should incorporate fundamental knowledge and be based on, and contribute to, the students’ development of a strong conceptual framework. Research comparing the performance of novices and experts, as well as research on learning and transfer, shows that experts draw upon a richly structured information base. Although factual information is necessary, it is not sufficient. Essential to expertise is mastery of concepts that allow for deep understanding. Such understanding helps the learner reformulate facts into useable knowledge. Developing a conceptual framework allows the individual to organize information into meaningful patterns and store it hierarchically in memory. Research on learning provides support for Brandwein’s recommendation for major conceptual schemes as the basis for teaching science. Further, contemporary research on learning supports my proposal to use the Standards as the basis for content, curricular coherence, and greater congruence between scientific inquiry and science teaching.
RESPONSES TO CRITICISM OF THE SCIENCE CURRICULUM
Recent criticisms of curricular coherence can be summarized in four themes: lack of challenging content, lack of instructional focus, inappropriate time to learn, and lack of horizontal and vertical connections of content. Criticisms about the lack of challenging content center on the overemphasis of facts and general lack of a conceptual orientation for science programs. For example, one can ask whether a curriculum is oriented toward scientific concepts that are fundamental to a discipline or myriad of topics that may be interesting, but do not emphasize scientifically fundamental concepts or processes. Lack of instructional focus refers to the lack of depth of treatment of content. For example, content may only receive superficial treatment in the curriculum. Inappropriate time to learn refers to the amount of time a concept remains in the curriculum. For example, some concepts are given very short and inadequate time, while others have a presence much beyond an adequate time for learning to occur. Finally, some concerns about coherence refer to the lack of connections among science concepts and inquiry abilities in both horizontal and vertical dimensions of the curriculum. I propose that the cumulative effect of these criticisms is lower student achievement, in particular on national and international assessments, but these qualities can be addressed as issues of curricular design.
To the question of challenging content, school science programs should be based on fundamental or essential scientific concepts and inquiry abilities. Documents such as the National Science Education Standards (NRC, 1996), Benchmarks for Science Literacy (AAAS, 1993), and TIMSS Assessment Frameworks and Specifications (ITEA, 2001) and the framework for the OECD-sponsored Program for International Student Assessment (PISA) (OECD, 2000) have answered questions about challenging content — — each provides a model for what students should know and be able to do. (See Figure 4 for an example from the national standards.)
Figure 4: Example of Challenging Content from the National Science Education Standards
Challenging content for the science curriculum centers on the conceptual orientation of the standards. Example from the National Science Education Standards: As a result of activities in grades K-4, all students should develop understanding of: Organisms and Their Environments |
However, identifying the content for school science is not enough. One must attend to other curricular and instructional issues. The design of programs must address focus, the depth of treatment for fundamental concepts, and procedures. Figure 5 presents an example of focus based on the national standards. Note that the statements include clearly delimited content for the science curriculum and assessments.
Figure 5: Example of Instructional Focus from the National Science Education Standards
Instructional Focus refers to the depth of treatment of content. ORGANISMS AND THEIR ENVIRONMENTS (Grades K-4) All animals depend on plants. Some animals eat plants for food. Other animals eat animals that eat plants. An organism’s patterns of behavior are related to the nature of that organism’s environment, including the kinds and numbers of other organisms present, the availability of food and resources, and the physical characteristics of the environment. When the environment change s, some plants and animals survive and reproduce, and others die or move to new locations. All organisms cause changes in the environment where they live. Some of these changes are detrimental to the organism or other organisms, whereas others are beneficial. Humans depend on their natural and constructed environments. Humans change environments in ways that can be either beneficial or detrimental for themselves and other organisms. |
From: National Research Council. (1996). National Science Education Standards, page 129.
Washington, DC: National Academy Press.
Appropriate time to learn is closely related to focus. The teaching of science requires one to not only ask what content should be in the curriculum, but the depth to which the content should be developed. One also must ask what is an appropriate amount of time for students at different developmental stages to learn the content. This is a measure of the opportunities for student learning in lessons, courses, and across the curriculum. I propose that the time to learn some content is quite short; for example, the basic idea that “All animals depend on plants. Some animals eat plants for food. Other animals eat animals that eat the plants” (NRC, 1996, p. 129) can be learned at an introductory level in a relatively short time, perhaps three or four 30-minute lessons. On the other hand, the basic idea that “An organism’s patterns of behavior are related to the nature of that organism’s environment, including the kinds and numbers of other organisms present, the availability of food and resources, and the physical characteristics of the environment” (NRC, 1996, p. 129) may take longer and require a spiraling through different grades with five or six experiences at different grade levels and with exposure for varying amounts of time.
Coherence refers to the number of concepts developed in a uniform set of experiences (for example, lesson, unit, and course) and within a school program (for example, elementary, middle, high school, and college). It is a measure of the connectedness among the science concepts that students experience during their study of science. Note that there are both horizontal (that is, across a course) and vertical (that is, between grade levels in school science programs) dimensions to curricula coherence. F. James Rutherford (2000) has written about coherence in high school programs. Rutherford states:
If coherence in high school science courses is a desirable property,
then one can reasonably argue that it should be present at every level
of content organization: lessons, units, courses, sequences of courses,
and entire curricula. Thus, the topics and activities making up a science
lesson or chapter ought to connect with one another to tell a (very limited)
story, with, as it were, a discernable beginning, middle, and end. Similarly,
the lessons or chapters making up a science unit should connect one
another in interesting ways to tell a complete (but still limited) story,
and units should connect with one another in interesting ways to tell a
more comprehensive story. Notice that two conditions must prevail at
each level of organization: All of the parts forming a unit or course must
be coherent, and all of those parts must join together to form a conceptual
whole (p. 22, 23).
For school science programs, achieving coherence will require curricular designs where less is more; that is, fewer concepts are studied in greater depth. Figure 6 uses the national standards as an example. In this figure horizontal coherence is modeled within a grade level for a key concept when you read down a column. Vertical coherence between grade level bands is modeled when you move from one column to the next.
Figure 6: Example for Horizontal and Vertical Coherence from the National Science Education Standards
Curricular coherence refers to the connections among concepts in both horizontal and vertical dimensions of the curriculum. | ||
Organisms and Their Environments (Grades K-4)
| Populations and Ecosystems (Grades 5-8)
| The Interdependence of Organisms (Grades 9-12)
|
National Research Council. (1996). National Science Education Standards. (pages 129, 157-58, 185).
Washington, DC: National Academy Press.
For many reasons, the teaching of science has lost coherence (Schmidt, et al 2001). Indeed, the U.S. curriculum has been analyzed against top achieving countries in the Third International Mathematics and Science Study (TIMSS) and was found to lack coherence in ways generally discussed in this essay (Schmidt, 2003).
The examples I used from the national standards demonstrated what might be meant by coherence. Although they served as adequate examples, they were not an actual science curriculum. I will use an elementary program developed by BSCS to show an actual curricular framework based on the national standards.
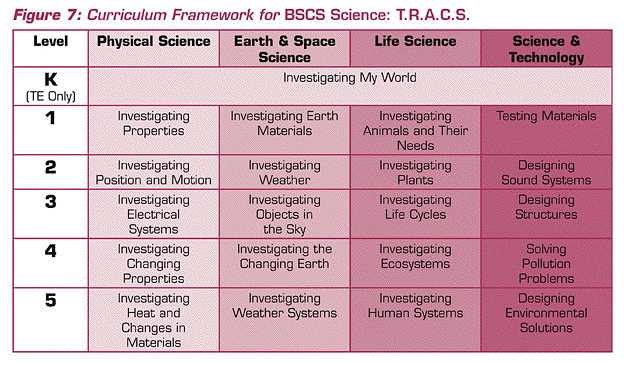
The program BSCS Science: T.R.A.C.S. serves as the example. As you can see in Figure 7, the program uses major content themes from the Standards and has a coherent vertical and horizontal curricular structure. Although the “grain size” of units differs from that proposed in the 1960s by Brandwein, the focus on major conceptual themes is consistent. I note the contrast of this BSCS program with many contemporary programs often “developed” locally that might be characterized as “patchwork quilts.” That is, the programs have a mixture of commercial units, textbook chapters, and assorted activities all forming a program. One other salient criticism of many contemporary programs resides in their lack of vertical coherence. Many school districts pay less attention to the organization and development of learning outcomes from grade to grade, compared to learning outcomes within grades. Let me turn to my third theme: congruence.
CONGRUENCE
What do I mean by congruence and the teaching of science? Congruence refers to a mode of teaching, what the science teacher does, and those tangible and intangible things that contribute to the teaching of science in any particular classroom. The term congruent means coinciding, agreeing, or corresponding. In the context of this essay, congruence parallels Brandwein’s use of the term style. What then would be congruence in the teaching of science?
In the broadest sense, congruence in the teaching of science means that the teacher brings the elements of content and curricular coherence together in ways that optimize learning for students. But this could be said for any discipline. I suggest that congruence in the sciences should center on the theme of inquiry. Teaching science as inquiry brings together content and pedagogy in ways that broaden and deepen student learning and ultimately the students understanding and appreciation of science.
In A History of Ideas in Science Education, George DeBoer (1991) states, “If a single word had to be chosen to describe the goals of science educators during the 30-year period that began in the 1950s, it would have to be inquiry” (p. 206). Although inquiry has been a goal for decades, that does not mean it has been implemented as an integral feature of school programs or science teaching. Indeed, a major synthesis of research published in the 1980s indicated that inquiry did not have a prominent role in science education (Welch, et al. 1981). This finding was especially disappointing because there had been discussion of inquiry by prominent individuals (Schwab, 1966; Rutherford, 1964) and programs that incorporated inquiry as a prominent theme. Like many goals, inquiry provides a rallying point of an apparent common agreement that fosters a sense of community and support among the advocates. Also common to most educational goals, there emerges the need for concrete examples of the abstract ideas and attitudes conveyed by the goal.
BSCS AND TEACHING SCIENCE AS INQUIRY
From the earliest days, BSCS has included inquiry in its programs (Randolph, 2002). Indeed, in the late 1950s, the deliberate, explicit, and comprehensive inclusion of “biology as inquiry” was a radical departure from other biology textbooks. At the time, H.J. Muller, a Nobel Laureate and BSCS Steering Committee member stated, “The trouble is not that there is too much science but too much short-sighted application of it, too little dissemination of its deeper meanings, and too little appreciation of the need for proceeding by its method of free inquiry.” (Muller, 1957, p. 252) Although scientists leading the work at BSCS supported the inclusion of inquiry, it was Paul Brandwein and Joseph Schwab that likely contributed the most to actually implementing the theme of science as inquiry. Schwab’s classic statement on the theme, his 1961 Inglis Lecture entitled “The Teaching of Science as Enquiry,” became a foundational statement for BSCS curriculum development (Schwab, 1966). He later discussed the theme in the Biology Teachers’ Handbook (Schwab, 1963).
The original BSCS programs used several avenues for implementing inquiry. First, the textbooks intentionally incorporated expressions that indicated the uncertainty and incompleteness of science and the possibilities that, through inquiry, the uncertainty might be reduced and the knowledge more complete. The texts had phrases such as “scientists are uncertain,” “we have been unable to discover the mechanism,” and “The favored theory is….” The phrases were designed to leave an accurate view of science and help students understand science as inquiry. Second, BSCS programs tried to replace “rhetoric of conclusion” with a “narrative of inquiry.” The textbooks included discussion indicating that science advances stepwise through investigations, experiments, data, and interpretations of data. Third, laboratory work was organized so it conveyed the sense that science, as the students experienced it, was inquiry. Although many laboratories were of the traditional type — that is, designed to help students understand a concept — some were truly investigatory. Students investigated questions for which the text did not provide an answer. Some laboratories in the texts, the supplemental laboratory blocks, and the aforementioned research problem series spearheaded by Brandwein were examples of this mode of inquiry in BSCS programs. Finally, there were “Invitations to Inquiry” that provided another means to implement science as inquiry in biology programs (Schwab, 1963).
BSCS implemented inquiry in biology courses through narrative, laboratories and invitations to inquiry. With time, the market’s influence on the revision of the original BSCS biology textbooks and declining support for the innovative NSF programs resulted in wider acceptance of conventional textbooks and decreased implementation of BSCS programs. Teaching science as inquiry became associated with doing laboratories; the primary aim of which was learning facts and information. By the early 1980s, adoption of BSCS programs was at low ebb. To be clear, the science teaching profession had lost sight of the richness of the inquiry theme by Schwab and Brandwein discussed. Science textbooks had moved inquiry to the background, and science teachers began equating inquiry with “hands-on” approaches. To state the situation directly, the goal of inquiry had been reduced to a few laboratories and a slogan. Ironically, it also was in the 1980s that research supporting the efficacy of teaching science as inquiry, and especially the effectiveness of BSCS programs began emerging (Shymansky et al, 1983, 1990).
In both of his 1960s essays, Paul F-Brandwein discussed scientific inquiry. In fact, Brandwein devoted considerable space to the various aspects of scientific investigation. Brandwein did not, however, propose inquiry as content. Rather, he assumed it as a strategy or part of the science teachers’ style. Just as it is important for students to understand major conceptual themes such as those described by Brandwein, I also suggest that it is important for students to develop the abilities and an understanding of scientific inquiry. I believe that developing an understanding of scientific inquiry is generally consistent with Brandwein’s views. He demonstrated in numerous discussions of science and scientists that he had a clear and deep understanding of inquiry. It seems only reasonable to recommend that any contemporary view of content would include scientific inquiry. Again, the Standards provide valuable information for answering questions about scientific inquiry as content and teaching style, to use Brandwein’s term.
A New Affirmation of Science as Inquiry
Publication of the National Science Education Standards in 1996 gave new life to the inquiry goal. The Standards presented inquiry as a prominent theme for both teaching and content. Here is a quotation from that document:
Scientific inquiry refers to the diverse ways in which scientists study the natural world and propose explanations based on the evidence derived from their work. Inquiry also refers to the activities of students in which they develop knowledge and understanding of scientific ideas as well as an understanding of how scientists study the natural world (NRC, 1996, p.28).
The actual standard states that “As a result of activities in grades 9-12, all students should develop both abilities necessary to do scientific inquiry and understandings about scientific inquiry.” Figures 8 and 9 present the fundamental abilities and understandings associated with these respective components of the standards.
Figure 8: Abilities of Scientific Inquiry
|
Figure 9: Understandings about Scientific Inquiry
|
The Standards shifted the implementation of the teaching-science-as-inquiry theme from an emphasis on “the processes” to cognitive abilities such as reasoning with data, constructing an argument, and making a logically coherent explanation. Further, the Standards made it clear that the aim of science education included students’ understanding of scientific inquiry. These are elaborated in INQUIRY and the National Science Education Standards (NRC, 2000), an addenda to the Standards that also includes a summary of research on inquiry.
SUMMARY AND CONCLUSIONS
This essay used Paul F-Brandwein’s themes of substance, structure, and style as points of departure for a discussion of content, coherence, and congruence as they relate to the teaching of science. For Brandwein, substance referred to major conceptual schemes of the sciences. Structure referred to the curricular organization; that is, how are those conceptual schemes organized and developed in the educational environment? Finally, he referred to style as the complex interactions in the classroom; in particular, what science teachers do to accomplish their goals. In essence, style is the teaching of science. Now one might ask, “What about the themes of this essay — content, coherence, and congruence?”
My themes establish contemporary perspectives for Brandwein’s themes. In a sense, I used the spirit of science by building on the past, by being appropriately skeptical, and by applying new ideas that complement and elaborate Brandwein’s original ideas.
Brandwein proposed six conceptual schemes for the science curriculum. These schemes were truly big ideas in the sciences. While sustaining the essential need for conceptual schemes as a central feature of science education, I argue there is a need for a broader range of content. Specifically, the content described in the National Science Education Standards (NRC, 1996) should be the basis for the science curriculum. This recommendation establishes consistent outcomes for science education while leaving open a variety of curriculum options, emphasis, and organizations. My recommendations only state that we agree upon the content outcomes, not the particular instructional materials that would be implemented to achieve those outcomes. To be clear, I am not making a recommendation that we impose a national curriculum. Mine is an argument to agree on ends, and it leaves the means of achieving those ends up to states and local jurisdictions.
Standards purposes that content not only include the physical, life, and Earth-space sciences; but also include inquiry, technology as it relates to science, personal and social perspectives, and the history and nature of science. We have curricula that are much too crowded with irrelevant information and trivial facts and have too little emphasis on the basic concepts and scientific inquiry. It is well past the time to achieve the proper balance of content in the science curriculum, and the national standards suggest an appropriate and meaningful balance.
Brandwein argued that the substance of science should not be the structure of school science curricula. My point here parallels his, namely that science content is not the science curriculum. In a contemporary perspective, the current curriculum experienced by many students is the metaphorical equivalent to watching television while another person has the channel selector and is clicking through the channels so quickly that the viewer only gets a glimpse of the storylines in dramas, scores in sports, recipes in cooking, sale items in home shopping, headlines in news, or guests on talk shows. Imagine then, taking a test on the stories, scores, recipes, sales, headlines, and guests. I believe you get my point about curricular incoherence. Comparing the U.S. science curricula with those of other high-achieving countries demonstrates just how incoherent our programs are. The results of international assessments such as TIMSS and PISA provide evidence about student achievement in the U.S. as compared to other countries.
In contrast, we should consider a few basic science concepts that have been defined in the Standards and use them as the central emphasis for the curriculum. This would help establish curricular coherence in science. I discussed several themes related to curricula coherence, challenging content, appropriate focus, time to learn, and horizontal and vertical connections. My recommendation centers on the need for developing and implementing instructional materials that contribute to greater curricular coherence. As a first step toward meeting this recommendation, I suggest using the Standards as the basis for curriculum development. I also noted the existence of some curriculum materials such as BSCS Science: T.R.A.C.S. and other BSCS programs that exemplify curricular coherence, especially when compared to many locally compiled programs.
My third theme was congruence. In the context of this essay, I argue that the teaching of science should be congruent with scientific inquiry. The era represented by Paul F-Brandwein’s contributions brought inquiry into science education, especially into the popular lexicon of science teachers and science educators. Most science teachers in the 1960s and 1970s would claim they were “inquiry teachers,” especially if they were using programs that have come to symbolize that era: BSCS Biology, PSSC Physics, CHEM Study, and ESCP Earth Science would be examples. In that era, “teaching science through inquiry” was commonly heard. Inquiry teaching became synonymous with using investigations or doing laboratory activities. Upon close examination, the investigations were designed to facilitate students’ learning of content. This was especially true for science teaching at the secondary level. Such use of investigations has its place and should continue to be a part of science teaching.
In addition to implementing of activities to enhance conceptual understanding of science, investigations also can be used to develop students’ abilities associated with scientific inquiry. The science teacher’s goal in this case shifts from an exclusive emphasis on content to facilitating reasoning by asking the student questions about, for example, possible explanations, the role of evidence, alternative explanations, and consistency of current scientific knowledge with students’ explanations.
In addition, the teaching of science should include the development of students’ understanding of inquiry and the nature of science. These are largely neglected outcomes of science education, yet citizens often encounter situations that require some understanding of science as a way of knowing, as a human endeavor with distinct processes that produce knowledge about the natural world.
Paul F-Brandwein’s works left the science education community with an intellectual investment that had the potential to grow significantly. The contemporary perspective I have tried to provided here shows how much we can still draw on his work. The teaching of science is even more important today than it was 40 years ago, because a sound understanding of science and technology has become essential to our society and the international community. We can take a major step toward improving the teaching of science for all students by systematically and effectively introducing challenging content, increasing curricular coherence, and implementing instructional congruence.
REFERENCES
American Association for the Advancement of Science (AAAS). (1993). Benchmarks for science literacy. New York: Oxford University Press.
Bardeen, M. & Lederman, L.L.. (1998). Coherence in science education. Science, 281 (10 July): 178-179.
Bass, H. (1998). The math education debates. Remarks developed from presentations to the
Center for Science, Mathematics, and Engineering Education, in February 1998, Beckman Center, Irvine, CA. Later expanded to the Research symposium — Reflecting on the math wars: Perspectives on the role of research and researchers in the public discourse about mathematics education reform in April 1998, NCTM meeting, Washington, DC.
Bransford, J., Donovan M.S., & Pelligrino, W. (Eds.). (1999). How people learn: Bridging research and practice. Washington D.C.: National Academy Press.
Bransford, J., Brown, A., & Cocking, R. (Eds.). (2000). How people learn: Brain, mind, experience, and school. Washington, DC: National Academy Press.
Brandwein, P. (1955). The gifted student as future scientist. New York: Harcourt Brace
Brandwein, P. (1962). Elements in a strategy for teaching science in the elementary school. The Burton Lecture. New York: Harcourt, Brace & World, Inc.
Brandwein, P., Metzner, J., Morholt, E., Roe A. & Rosen, W. (1962). Teaching high school biology: A guide to working with potential biologists. Biological Sciences Curriculum Study Bulletin No. 2. Washington, DC: American Institute of Biological Sciences. Jovanovich, Inc.
Brandwein, P. (1965). Substance, structure, and style in the teaching of science. New York: Harcourt Brace Jovanovich, Inc.
Brandwein, P. (1972). The permanent agenda of man: The humanities. New York: Harcourt Brace Jovanovich, Inc.
BSCS. (1959, September). Personnel. BSCS Newsletter, 1, 5.
BSCS. (1960, May). BSCS committee plans program of original investigations for science-prone students. BSCS Newsletter, 3, 3.
BSCS. (1961, April). Biological Investigations for secondary school students. BSCS Newsletter, 7, 4.
BSCS. (1963). Biology teachers’ handbook. (Schwab, J. Ed.). New York: John Wiley and Sons, Inc.
Bybee, R. (1997). Achieving scientific literacy. Portsmouth, NH: Heinemann.
Conant, J.B. (1957). (Eds). Harvard case histories in experimental science. Cambridge: Harvard University Press.
DeBoer, G. (1991). A history of ideas in science education. New York: Teachers College Press.
Glass, B. (1976). Reflections on the early days of BSCS. BSCS Newsletter, Vol. 64, 1-3.
Muller, Hermann, J. (1957). Man’s place in living nature. Scientific Monthly, 84: 245.
Mullis, I.V., Martin, M., Smith, T., Garden, R., Gregory, K., Gonzalez, E., Chrostowshi, S., O’Connor, K. TIMSS assessment frameworks and specifications 2003. Boston: International Association for the Evaluation of Educational Achievement (1EA).
Kliebard, H. (1994). Curriculum ferment in the 1890s. In M. Cobb (Ed.), The future of Education: Perspectives on national standards in America. New York: College Entrance Examination Board.
Metzenberg, S. (1998, July 23). Testimony. Hearing before the United States House of Representatives Committee on Science, Subcommittee on Basic Research.
National Research Council (NRC). (1996). National science education standards. Washington, DC: National Academy Press.
National Research Council (NRC). (2000). Inquiry and the national science education standards. Washington D.C.: National Academy Press.
OECD. (2000). Measuring student knowledge and skills: The PISA 2000 assessment of reading, mathematical, and scientific literacy. Paris: Organization for Economic Co-Operation and Development.
Olson, S. (1998). Science friction. Education Week, September 30. 25-29.
Randolph, J. L. (2002). Scientists in the classroom, New York: Palgrave.
Rutherford, F. J. (1964). The role of inquiry in science teaching. Journal of Research in Science Teaching, Vol. 2: 80-84.
Rutherford, F. J. (2000). Coherence in high school science. In Making sense of integrated science: A guide for high schools. Colorado Springs, CO: BSCS.
Schmidt, W.H. & Knight, C.C. (1998). What can we really learn from TIMSS? Science, 282, 1830-1831.
Schmidt, W.H. McKnight, C.C., Houang, R.T., Wang, H.C., Wiley, D.E., Cogan, L.S. & Wolfe, R.G. (2002). Why schools matter: A cross-national comparison of curriculum and learning. San Francisco: Jossey-Bass.
Schwab, J. (1961). Some reflections on science education. BSCS Newsletter, September, No. 9: 8-9.
Schwab, J. (1962). The concept of the structure of a discipline. The Educational Record, July: 197-205.
Schwab, J. & Brandwein P. (1966). The teaching of science. Includes The Inglis Lecture, The teaching of science as enquiry by Joseph J. Schwab, and The Burton Lecture, Elements in a strategy for teaching science in the elementary school, by Paul Brandwein. Cambridge, Massachusetts: Harvard University Press.
Stillman, C. (1997). Science in an ecology of achievement. The First Paul F-Brandwein Symposium, Dingmans Ferry, Pennsylvania. 13-17 November.
Welch, W., Klopfer, T., Aikenhead, G., & Robinson, J.. (1981). The role of inquiry in science education: Analysis and recommendations. Science Education, Vol. 65: 33-50.
